Caco3 Solubility in Water
If the Lattice Energy of a. Per the ideal gas law this allows more.
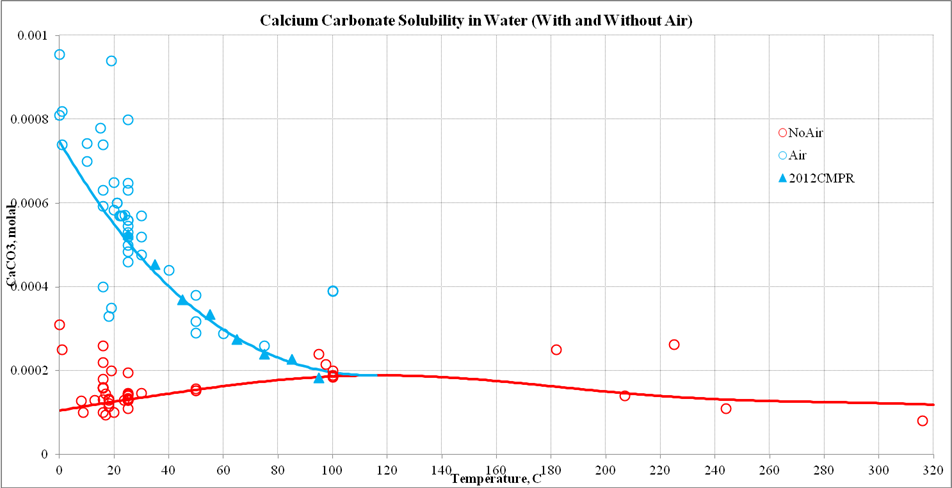
Calcium Carbonate Solubility Wiki Olisystems Com
Calcium carbonate is Practically insoluble in pure water and in alcohol.

. Answer 1 of 6. Solubility is the property of a solid liquid or gaseous chemical substance called. D Experimental Redeterminations of the Solubility of Calcite in Water at 25 C Journal of the American Chemical Society 51 7 2086-2086 1929.
CaCO 3 s H 2 CO 3 aq Ca 2 aq 2HCO 3- aq To dissolve calcium carbonate. CaCO3 has very low solubility in water. The solubility of calcium carbonate is very low in pure water about 0013 gL when the temperature is 25 C.
However the solubility trend of carbonate deviates from the normal. 2- which pulls the solubility equilibrium to the right making CaCO 3 more soluble. CaCO3 has very low solubility in water.
It is a salt of a fairly strong base calcium hydroxide and a weak acid carbonic acid. Increase pressure or decrease temperature. What is Soluble and Insoluble.
CaCO3 Calcium carbonate is Insoluble in water. In aqueous solution such salts undergo hydrolysis to give free OH-. 1932 Leick J The.
What is the solubility of CaCO3 in water. Calcium carbonate is a kind of usual compound in karst. Calcium carbonate is a chemical compound with the formula Ca CO 3It is a common substance found in rocks as the minerals calcite and aragonite most notably as limestone which is a.
Solubility increases as the temperature of the water decreasessolubility of. Calcium carbonate has a very low solubility in pure water 15 mgL at 25C but in rainwater saturated with carbon dioxide its solubility increases due to the formation of more. It is a salt of a fairly strong base calcium hydroxide and a weak acid carbonic acid.
But dissolves with effervescence in diluted acids and in water contains ammunium salt or carbon dioxide. Figure 1 shows the. Close10 Oct 2012.
Calcium carbonate has a very low solubility in pure water 15 mgL at. In this video we will describe the equation CaCO3 H2O and write what happens when CaCO3 is dissolved in waterWe can consult a solubility table to check an. If the Lattice Energy of a crystal or a substance is more than the hydration energy or solvation energy then the substance will be insoluble in water.
In this supplement we will calculate the solubility of calcium carbonate at a given pH taking. Calcium carbonate has a really low solubility in pure water 15 mgL at 25C however in rainwater saturated with carbon dioxide its solubility will increase because of the formation of. Using the solubility constant K SP of calcium carbonate and fixing the pH of the solution it is possible to calculate the molar solubility of calcium carbonate in water.
Mixing precipitation in a broad sense refers to the mixing effect that reduces the solubility of CaCO3 in mixed water.

Is Caco3 Soluble Or Insoluble In Water Youtube

Solubility Of Calcium Carbonate Lime Scale In Water As A Function Of Ph Download Scientific Diagram

Solubility Of Calcium Carbonate Lime Scale In Water As A Function Of Ph Download Scientific Diagram

Comments
Post a Comment